Statistical Consulting
SynoloStats collaborates with manufacturers of all sizes, from small virtual biotechs to the largest, global pharmaceutical manufacturers, across all product platforms.
Support can vary from a short-term collaboration such as a response to a regulatory question requiring statistical expertise, to more extensive support for the product and process evidence required for a BLA or NDA submission.
Solutions incorporate relevant ICH and regulatory agency guidance and requirements along with business goals and constraints.

Lifecycle Stage
Process Design
Develop Robust Processes and Control Strategy
(both manufacturing and analytical)
Statistical Consulting Services
- Quality by Design
- Flowdown from CQAs to CPPs and CMAs to control strategy
- Design of Experiments
- Screening Designs, Optimal Designs, Factorial, Response Surface and Mixture for Formulations
- Design Space
- Multidimensional region to assure quality
- Specification Derivation and Acceptance Criteria
- Performance Based, Compendial and Clinically Derived
- Shelf-Life Determination and Stability Modeling
- Predictive Modelling
- Bayesian for complex data structures
- Assay Validation
- Method Robustness DOE
- Comparability Protocols
PPQ/PV
Demonstrate Quality
- The “Goldilocks” sampling plan
- Collecting evidence that the “next” dose will meet specification
- Risk based approach to the number of PPQ batches
- Avoiding failures from unnecessary statistical criteria
- Equivalence testing
- Rank order sources of variability
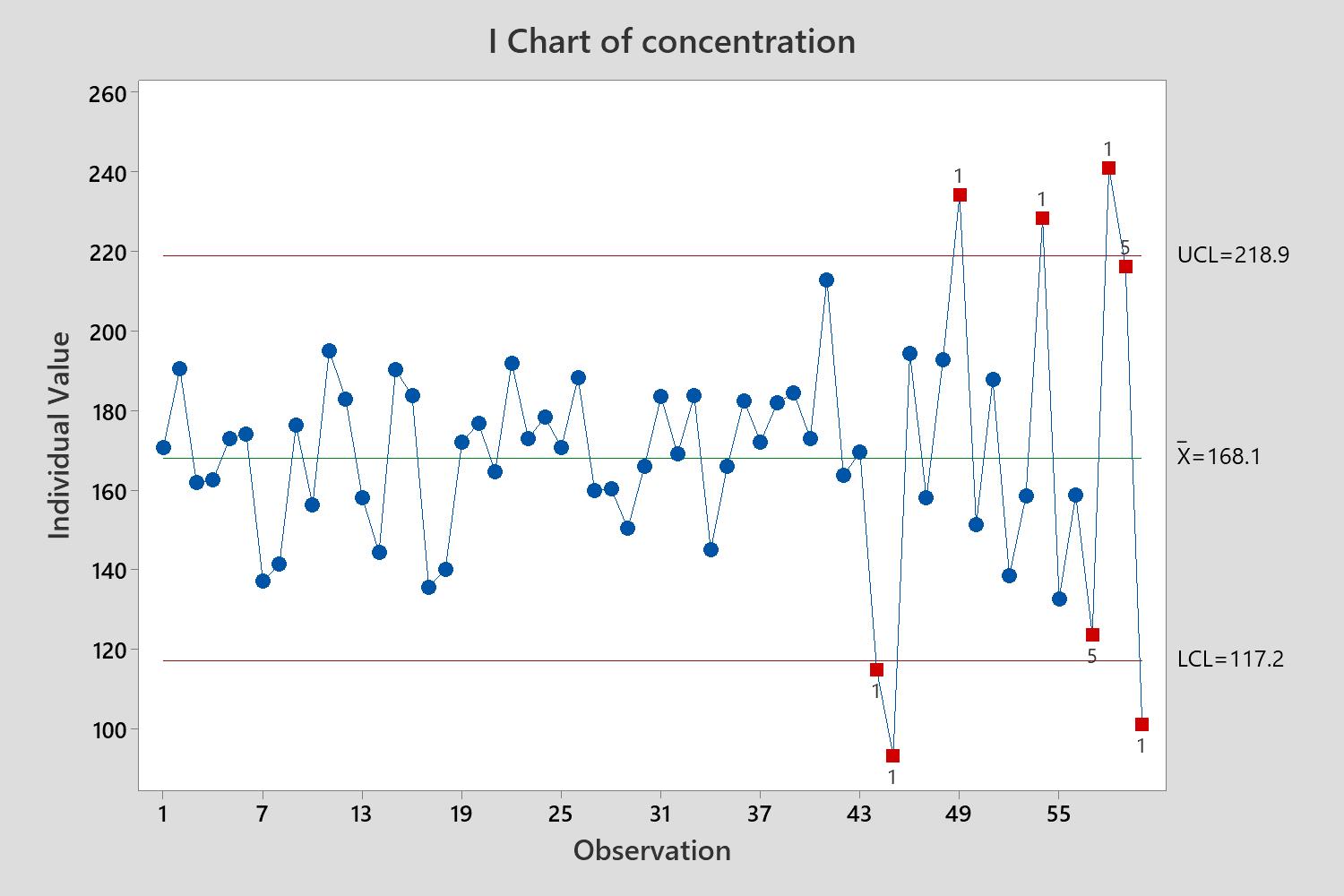
CPV/OPV
Assure Ongoing Quality
- The What When and How of a CPV program
- Trend analysis using control charts
- Risked based response to process changes
- Nuances of pharmaceutical data
- Process capability metrics i.e. Ppk
- Lean CPV
- CPV reporting
- Combining data visualization with Is/Is Not analysis to speed investigations

Your Statistics Partner for Pharmaceutical Process Development and Manufacture
Copyright © Synolostats. All rights reserved. Designed By WD
